Acids and Bases: Lewis vs. Bronsted
Acids and bases play vital roles in many organic chemical reactions. This page examines two complementary definitions of acids and bases, and explores some of the more common ways in which acids and bases are involved in promoting organic reactions. The factors that influence the relative strengths of acids and bases are also reviewed.
Strongly Related Topics
- Physical Properties of Organic Compounds
- Energy Diagrams: Describing Chemical Reactions
- Resonance and Resonance Forms: Conjugation and Conjugated Systems
- Isomers/Isomerism
Somewhat Related Topics
| acidity constant | Bronsted-Lowry acids | Bronsted-Lowry bases |
| conjugate acid | conjugate base | electrophile |
| HOMO | Lewis acid | Lewis base |
| LUMO | nucleophile | resonance effect |
Acids and Bases: Lewis vs. Bronsted
There are two complementary definitions of acids and bases that are important:
- the Bronsted (or Bronsted-Lowry) definition: an acid is a proton (H+ ion) donor, and a base is a proton acceptor;
- the Lewis definition: an acid is an electron acceptor, and a base is an electron donor.
Fig. 1


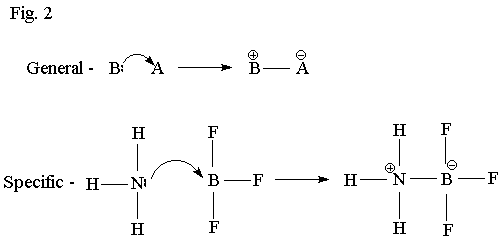
Self-Test Question #1 Below are shown two very similar acid/base reactions:

In each case, decide which of the reactants is a base, and which is an acid.
Also determine which process is a Lewis acid/base reaction, but is not a
Bronsted-Lowry acid/base reaction.
Links to Related reading in textbook (McMurry, Organic Chemistry, 4th ed.)
- Chapter 2, Section 2.6: pages 50-59
- Chapter 20: pages 774-801
- Chapter 24, Section 24.4: pages 941-945
Links to Related Computer-based Learning Materials
- Links to Related Chem TV Files
- Vol.1,Topic 4: Functional Groups
- Vol.2,Topic 4: Carboxylic Acids/Derivatives
- Vol.2,Topic 5: Amines
- Links to Related xxx Files
- Links to Related yyy Files
- Links to Related zzz Files
- Related Beaker Menu Fuctions
- Struct, "Functional Groups"
- React, "Hydrogen pKa"
- React, "Perform a Reaction"
Links To Related Internet Resources
Send questions, comments, or suggestions to:
Dr. Thomas H. Eberlein
the1@psu.edu
Copyright © 1996 Thomas H. Eberlein
Version 1.2.5, 3/17/97