
John Frederic
Daniell
b. March 12, 1790, London, UK
d. March 13, 1845, London, UK
 |
John Frederic Daniell was British chemist and meteorologist who invented the Daniell cell, which was a great improvement over the voltaic cell used in the early days of battery development. |
John Frederic Daniell was born in London, England, on 12 March
1790 as the son of a lawyer. He received a good education, while
attending private school. He received either an earned or a
honorary degree from Oxford University.
 |
Upon completing school with a good background in technology, he went to work for a relative who owned a sugar refinery. While working in the refinery he improved its technological operations and processes. After awhile he left the refinery to enter the field of education and research by taking a position as professor of physics at the University of Edinburgh, Scotland, when just 23 years of age. In 1823, he was elected as a Fellow in the Royal Society of London. In addition to his classes in physics he also worked as a chemist at the university, and began research in meteorology. Concurrent with his university responsibilities he successfully managed the Continental Gas Company in 1817. Daniell not only excelled because of his ability to make useful observation, classifications, and improvements in the physical sciences of his time; but he also demonstrated skill for scientific manufacturing enterprises with the development of a new process for general gas. |
 |
His research in 1820 led to the invention of a dew-point hygrometer that measured relative humidity which afterwards became a standard instrument. His hygrometer was made with two thin glass bulbs that were hung from a base and joined with a glass tube. One of the glass bulbs held ether and a thermometer that collected and dissipated dew when the other bulb was slowly cooled and reheated. The condensing temperature was produced by evaporation of the ether. Daniell's hygrometer, as it was called, enabled the easy determination of vapor that existed in a given mass of atmosphere. The average temperature recorded by the device was the dew point. In 1823, he published Meteorological Essays which soon became a popular book. This work is "the first attempt to collect scattered facts on the subject, and to explain the main phenomena of the atmosphere by physical laws. Daniell insisted on the paramount importance of extreme accuracy in meteorological observation, and kept a model record of atmospheric changes. He called attention to the necessity of attending to the moisture of hothouses, and caused a revolution in hothouse management." In a later edition he also discussed the meteorological effects of solar radiation and the cooling of the Earth. Daniell's Essay on Artificial Climate Considered in Its Applications to Horticulture showed the importance of humidity in greenhouses. |
Interest in the atmosphere enabled Daniell to improve hothouse plant growing. He really revolutionized hot house management by stressing the value of atmospheric moisture. Daniell was the first to use physical laws in trying to explain the phenomenon of atmosphere. His work leading to improvements in hothouse management earned him the silver medal award from the Horticultural Society in 1824.
In 1830, he invented a pyrometer that was successful in measuring heat. Daniell found that in fusing metals a liquid is constantly moved by the cooling of its exterior. His research in chemistry enabled him to produce gas from resin and measure very high temperatures. He also invented a water barometer in 1830.
He was the first professor of chemistry and meteorology at the then new King's College of London, and worked there from 1831 to 1845. While at King's College he was responsible for establishing a department of applied science. In 1832, he was awarded the Rumford Medal of the Royal Society of London for his earlier work with the pyrometer.
Daniell, however, is best known for
his work in electrochemistry. Asimov claims that his
interest in electrochemical research was encouraged by the work
of his friend Faraday.
 |
 |
Then in the early 1830s, Daniell became deeply interested in the work of his friend Michael Faraday so turned to electrochemistry for his main research interest at that time. A major problem with the Volta pile was that it could not provide current for a sustained period of time. Sturgeon worked on the problem when in 1830 produced a battery with longer life than that of Volta by amalgamating the zinc. Contributing to the major problem with batteries was a thin film of hydrogen bubbles that formed over the positive electrode. The thin film of hydrogen caused increased internal resistance of the battery that reduced its effective electromotive force (voltage). This process of a thin film of hydrogen collecting on the electrode is known as polarization.
Daniell began experiments in 1835 in an attempt to improve the Voltaic battery with its problem of being unsteady and as a weak source of electrical current. His experiments soon led to remarkable results. In 1836, he invented a primary cell in which hydrogen was eliminated in the generation of the electricity. Daniell had solved the problem of polarization. In his laboratory he had learned to alloy the amalgamated zinc of Sturgeon with mercury. His version was the first of the two-fluid class battery and the first battery that produced a constant reliable source of electrical current over a long period of time. That is, the power remained constant with this type of battery upon repeated application without removing the metals which was a source of weakness in all single fluid batteries. Until now the current of other batteries declined rapidly. His placement of a barrier between the copper and zinc plates stopped the hydrogen from forming.
The Volta battery (pile) emitted free hydrogen by the
electrolyte which then migrated to the positive copper pole. The
hydrogen accumulated on the pole to form a barrier that soon
stopped the flow of the current. Both single fluid and two-fluid
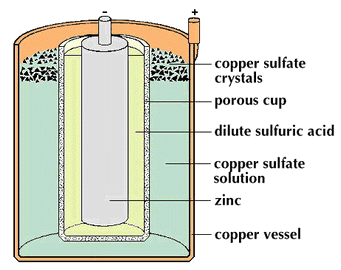
batteries used solutions to create the electricity. Daniell's
battery consisted of a cylindrical copper vessel that served as
the passive plate (pole). Placed inside the outer copper vessel
was a porous earthenware container or partition that held a zinc
rod or active plate (pole). The space between the copper and the
porous cup was filled with a solution of copper sulfate which was
kept saturated by crystals of the salt lying on a perforated
shelf. The porous cup was filled with dilute sulfuric acid. The
porous earthenware kept the fluids from mixing without hindering
the passage of current; it allowed ions to move through while the
reaction of the cell was taking place. The contents of the
battery had to be dismantled when not used to stop the chemical
reactions and conserve the metals. The sulfate of copper that was
in contact with the passive plate served to take up hydrogen. The
amalgamated zinc rod (anode) had a binding screw. The top of the
copper cylinder contained the other binding screw (cathode).
 A scheme of Daniell's battery - does not correspond to the real structure of it. |
The chemical reaction within the battery consisted of a decrease of zinc and an increase of copper; the zinc crowded out copper from its sulfate so that the copper sulfate continuously changed into zinc sulfate by replacement. Beard and Rockwell expressed the chemical reaction with the equation: Zn + H2SO4
+ CuSO4 = |
 |
The sulfuric acid was kept in the porous cup to prevent it from continuously acting on the copper and the zinc, and to keep the sulfate of zinc formed from contacting the copper. Since copper sulfate solution is heavy, it remained on the bottom of the cell. Daniell's battery with modifications had an operating voltage (i.e., gave constant electromotive force and retained a nearly constant internal resistance) of 1.11 volts. |
  |
Daniell's nattery was called a constant battery because it did not evolve gas, and therefore did not polarize, supplying a constant current. It furnished the unit of electric potential, the volt, as a column of mercury did the unit of resistance, the ohm. The Daniell cell still used the familiar copper and zinc electrodes. The zinc electrode was put in a cup of unglazed earthenware and bathed in dilute sulphuric acid. The copper was surrounded by crystals of copper sulphate that maintained a saturated solution. Instead of releasing hydrogen, the electrons were furnished to the copper ions in the electrolyte, which plated out as copper metal on any nearby surface. The purpose of the cup was to keep the solutions separate while allowing electrical conduction by ion migration. If the solutions mixed, local action ruined the battery. When the cell furnished current, the zinc dissolved to form zinc sulphate solution, and copper from the copper sulphate plated out on the copper electrode. |
No gases were involved at all, so the cell did not polarize. The cell has a fairly large internal resistance, but this was not a serious defect in view of the small currents required, and actually proved an advantage in many applications. It also protected the cell against damage if shorted. The copper sulphate even kept algae under control. However, the porous cup, intended to keep the solutions separate, was rendered impervious after a time by deposition of copper on it as the cell operated.
This internal resistance varied slightly with areas of the
copper and zinc plates immersed in the solutions, distance
between the metal plates, and the width and materials of the
walls of the porous cup. Its operating voltage depended on the
densities of the copper and zinc sulfate solutions. The operating
voltage increased (e.g., 1.14 V) by increasing the density of
copper sulfate solution, and its voltage decreased (e.g., 1.08 V)
by increasing the density of the zinc sulfate solution. When the
battery was not in use corrosion of the zinc plates was high
which greatly limited its longevity. His battery required little
maintenance, and did not give off noxious fumes. The Daniell
battery was less expensive than existing batteries. In 1837 (or
1836), he received the Copley medal from the Royal Society of
London for his work with the constant pile.
 |
An illustration showing the inside of a rural telegraph station with a female operator. The depiction of the telegraphic equipment is accurate - on the operator's table are a telegraphicc relay, a printing register, a cutoff switch, and a camelback telegraph key. On the floor is a battery box containing two Daniell cells, which supplies the electricity for the telegraph. |
 |
| The Daniell Battery. This combination consists of a jar of glass or earthenware, F (Fig. 3), about six inches in diameter and eight or nine inches high. A plate of copper, G, is bent into a cylindrical form, so as to fit within it, and is provided with a perforated chamber, to contain a supply of sulphate of copper in crystals, and a strap of the same metal with a clamp for connecting it to the zinc of the next element. H is a porous cup, as it is technically termed, made of unglazed earthenware, six or seven inches high and two inches in diameter, within which is placed the zinc, X. This is usually of the shape shown in the figure, which is called the ``star zinc,'' but it is often made in the form of a hollow cylinder, the latter giving greater power, but being somewhat more difficult to clean. The outer cell is filled with a saturated solution of sulphate of copper (blue vitriol), and the porous cell with a solution of sulphate of zinc. A series of three elements connected together, as usually employed on American lines for a local battery, is shown at I. |
 |
Daniell's last work on a gravity type of battery was later to become one of the most popular in the 1850's. He fused two electrolytes; copper sulfate (CuSO4) and zinc sulfate (ZnSO4). A copper electrode was placed in the bottom half of a glass battery jar, and then copper sulfate was added in crystal form. Next the zinc sulfate solution was floated on top of the copper sulfate. This approach decreased the need for a porous ceramic diaphragm to separate the two electrolytes, and decreased the internal resistance of the system. When the circuit was opened and let standing while open the copper ions would diffuse upwards and self discharge onto the zinc anode which resulted in loss of power. The operator added copper sulfate crystals to maintain a constant saturated solution that then could constantly produce its current. |
Daniell's research into development of constant current cells took place at the same time (late 1830s) that commercial telegraph systems began to appear. Early telegraph messages were brief and traveled short distances. Crude, weak batteries were sufficient to support the signal. With the increase in traffic and introduction of Morse sets, stronger currents and more constant output were required in the batteries. Daniell's copper-depolarized battery (1836) and Grove’s nitric acid depolarized cell were fortuitous arrivals. British and American telegraph systems used the Daniell cell exclusively, as it was the only one capable of being rapidly depolarized. His cells also produced a more constant output and generated a stronger current than Sand batteries. This was the "pre-volt" period, when the intensity of pain was used as a measure of a cell's power. The Daniell cell was widely used in France before the Leclanché cell was invented in 1868.
In 1839, Daniell experimented on the fusion of metals with a 70-cell battery. He produced an electric arc so rich in ultraviolet rays that it resulted in an instant, artificial sunburn. These experiments caused serious injury to Daniell’s eyes as well as the eyes of spectators. Ultimately, Daniell showed that the ion of the metal, rather than its oxide, carries an electric charge when a metal-salt solution is electrolyzed.
Daniell published the Introduction to the Study of Chemical Philosophy in 1839. In 1841, he became a founding member and vice president of the Chemical Society of London. He also authored many papers that were published in journals of science.
While conducting research in electrolysis he corrected an error on ions made earlier by Faraday. He found that acids and bases were not ions, and that only metals or metal-like compounds and halogens and groups of atoms of compound acids are ions.
Daniell was an illustrator, social philosopher, teacher, and writer. Daniell married and was the father of five daughters and two sons. While either attending a council meeting or delivering a lecture at the Royal Society on 15 March 1845 he had a heart attack and died at the age of 55 years. At the time of his death he was the Foreign Secretary (1839-1845) of the Royal Society.
![]() This text has been
compiled from the biographies of Daniell available in
the Internet:
This text has been
compiled from the biographies of Daniell available in
the Internet:
( 1,
2,
3, 4,
5,
6,
7,
8,
9,
10,
11
)