 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |

|
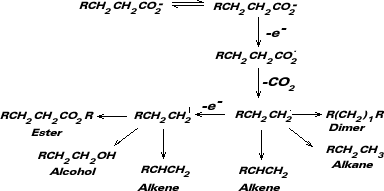
Sonoemulsion Kolbe Electrosynthesis [1,2] The Kolbe reaction is the electrosynthesis of hydrocarbons via electrooxidation of carboxylic acids [3]. Until now, monophasic Kolbe electrosyntheses have been exclusively studied, yielding a general mechanism: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biphasic Kolbe galvanostatic electrosynthesis was undertaken at both platinum and boron-doped diamond electrodes, the latter to minimise cavitation induced damage of the working anode surface. Two liquid aliphatic acids, hexanoic and heptanoic acids were investigated as model compounds that undergo Kolbe reactions. Each acid was co-emulsified with 1.0 M NaOH, and after the passage of one-Faraday-per mole charge (ca. 1500 oC), the reaction quenched and the products analysed using GC/MS and 1H NMR. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The dimer yield was found to depend upon the reaction temperature, the aqueous electrolyte concentration (and thus conductivity of the sono-emulsion), and amount of charge passed; the maximum observed yield was found to be 75% (comparable to monophasic electrolyses), with a current efficiency of 45%. Significantly, in contrast to monophasic electrolyses (where carbon-based anodes give 'two-electron' products) [4], the dimer yield in this electrosynthesis is anode material independent! Additionally, only one side product can be observed (in a yield typically less than 5%): the ester, amyl caproate (from hexanoic acid electrolysis), or capryl enanthoate (from the electrolysis of heptanoic acid). The fact that no other side products are formed suggests that R+ or R. are formed in a non-ion-conducting organic phase adjacent to the electrode surface. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A possible reaction mechanism is: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schematic drawing of the conditions during the formation of an insoluble product (organic deposit shown in grey) at the electrode surface under sono-emulsion reaction conditions. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
References |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| [1] J. D. Wadhawan, F.
Marken, R. G. Compton, S. D. Bull, S. G. Davies, Chem. Commun.,
2001, 87. [2] J. D. Wadhawan, F. J. Del Campo, R. G. Compton, F. Marken, S. D. Bull, S. G. Davies, D. J. Walton, S. Ryley, J. Electroanal. Chem., in press. [3] H. Kolbe, Ann. Chim., 1849, 69, 257. [4] C. J. Brockman, Electroorganic Chemistry, Wiley, New york, 1926. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||